
Program description
Anti-CCR8 depleting antibody
- Discovery
- Candidate
- Preclinical
- Phase I
DT-7012: a best-in-class Treg-depleting anti-CCR8 antibody pioneering a new era in GPCR-targeting immunotherapies
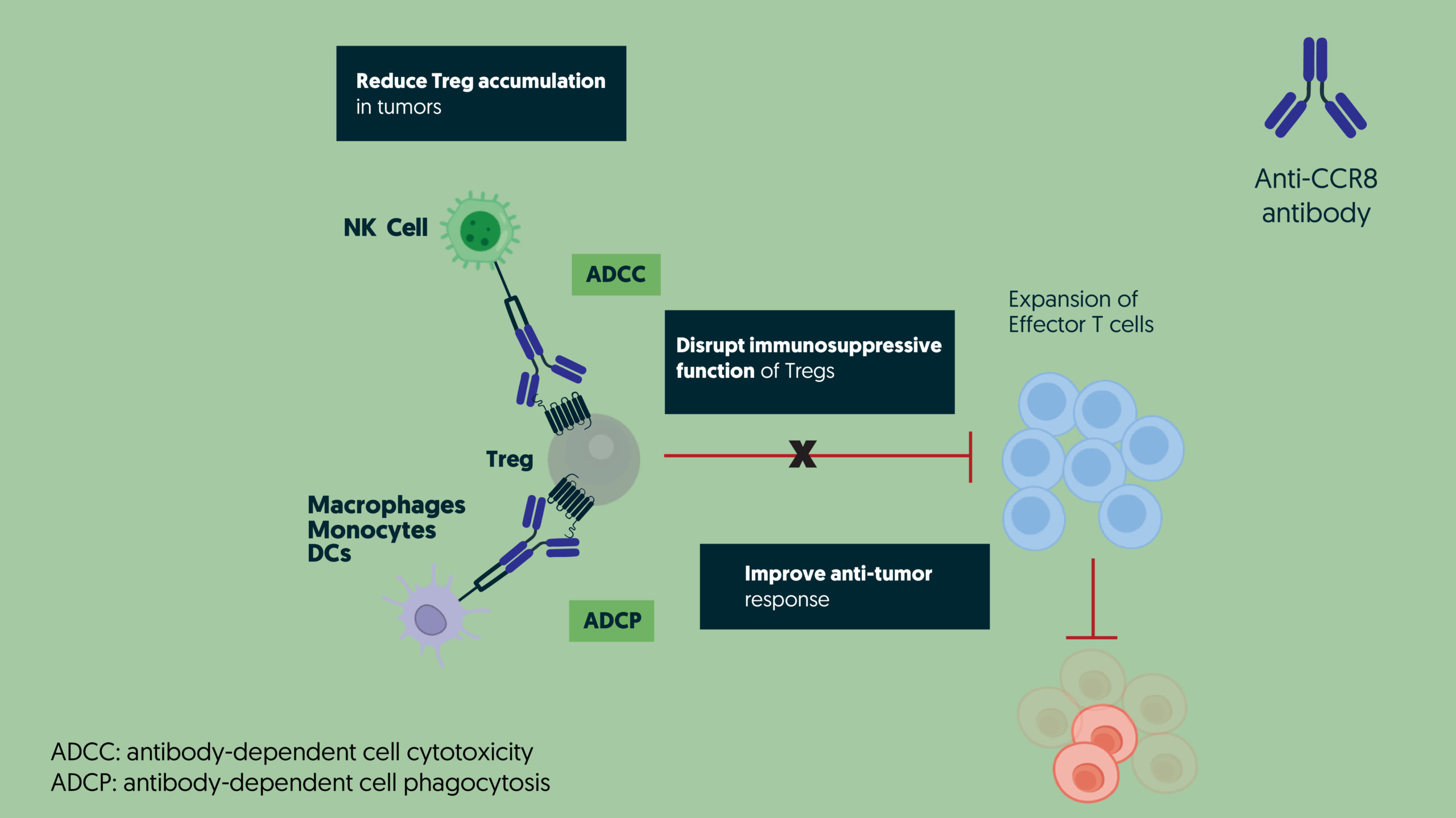
DT-7012, a differentiated anti-CCR8 monoclonal antibody, is a unique development in cancer immunotherapy. It has exceptional potential to preferentially deplete tumor-infiltrating regulatory T cells ( Tregs ) within the tumor microenvironment ( TME ) through antibody-dependent cellular cytotoxicity ( ADCC ) and antibody-dependent cellular phagocytosis ( ADCP ) mechanisms. By targeting these Tregs, DT-7012 effectively transforms the TME into an immunocompetent environment, promoting tumor regression and offering new hope to patients who have not responded to existing immunotherapies.
Currently in the pre-IND stage, DT-7012 is progressing in CMC (Chemistry, Manufacturing, and Controls) development to support Phase I clinical trials expected to begin in mid 2025. These trials will focus on assessing the safety, toxicity and initial efficacy of DT-7012 in patients with resistant cancers.
The multiple and strong patent protection of this asset highlights the broad therapeutic application of our Treg-depleting ADCC/ADCP antibodies, particularly DT-7012, solidifying our leadership in GPCR-targeting immunotherapies.
DT-7012: advancing precision science with transformative immunotherapy
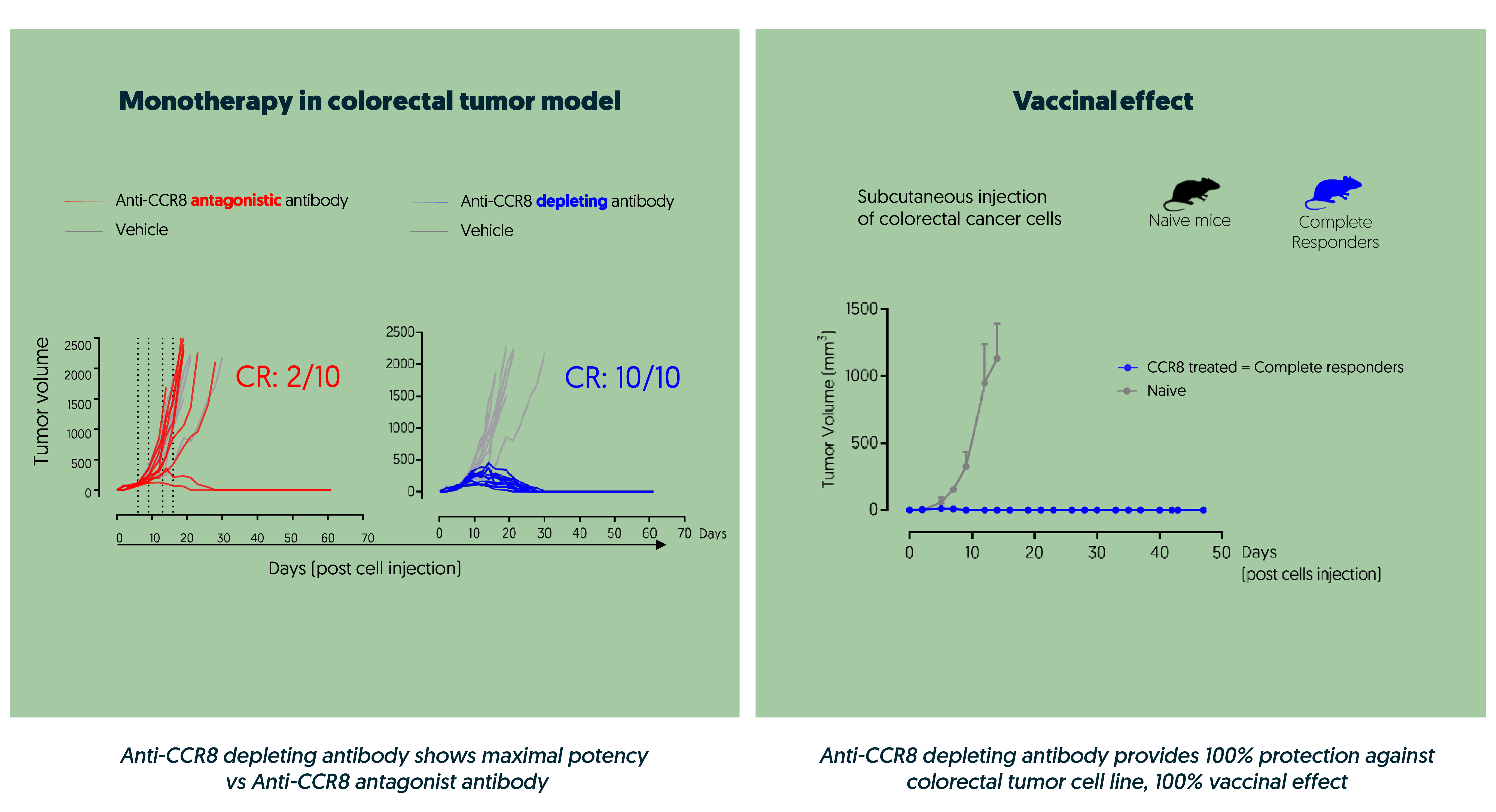
Through our precision-based approach and rigorous in vitro benchmarking against leading CCR8 antibodies, we have discovered a unique collection of proprietary anti-CCR8 antibodies, each with distinct and differentiated profiles. Among these, DT-7012, stands out as our best-in-class, differentiated Treg-depleting anti-CCR8 monoclonal antibody. Selected in 2023 for its unique ability to achieve 100% complete response as a preclinical monotherapy, DT-7012 represents a significant breakthrough in cancer treatment.
To support the development of DT-7012, Domain has developed a comprehensive translational research strategy that leverages an in-depth understanding of CCR8 pharmacology and precise biomarker analysis. This approach informs the design of first-in-human clinical studies, de-risking and increasing trial efficiency.
DT-7012’s unique therapeutic potential has been recognized by the prestigious Hospital-University Research in Health (RHU) SPRINT consortium, which is supporting its advancement to clinical trials by mid-2025 for the treatment of Cutaneous T-Cell Lymphoma ( CTCL ), a rare and currently incurable cancer that severely affects patients’ quality of life. This recognition not only validates DT-7012’s scientific foundation but also highlights its promise of addressing a significant medical need.
Differentiating properties of DT-7012: best-in-class potential with unique positioning
DT-7012 exemplifies our commitment to precision science and innovation within the GPCR field. By selectively depleting tumor-infiltrating Tregs, DT-7012 amplifies the activity of immunocompetent cells such as Dendritic cells, NK cells and CD8 T cells, improving the overall immune response against tumors.
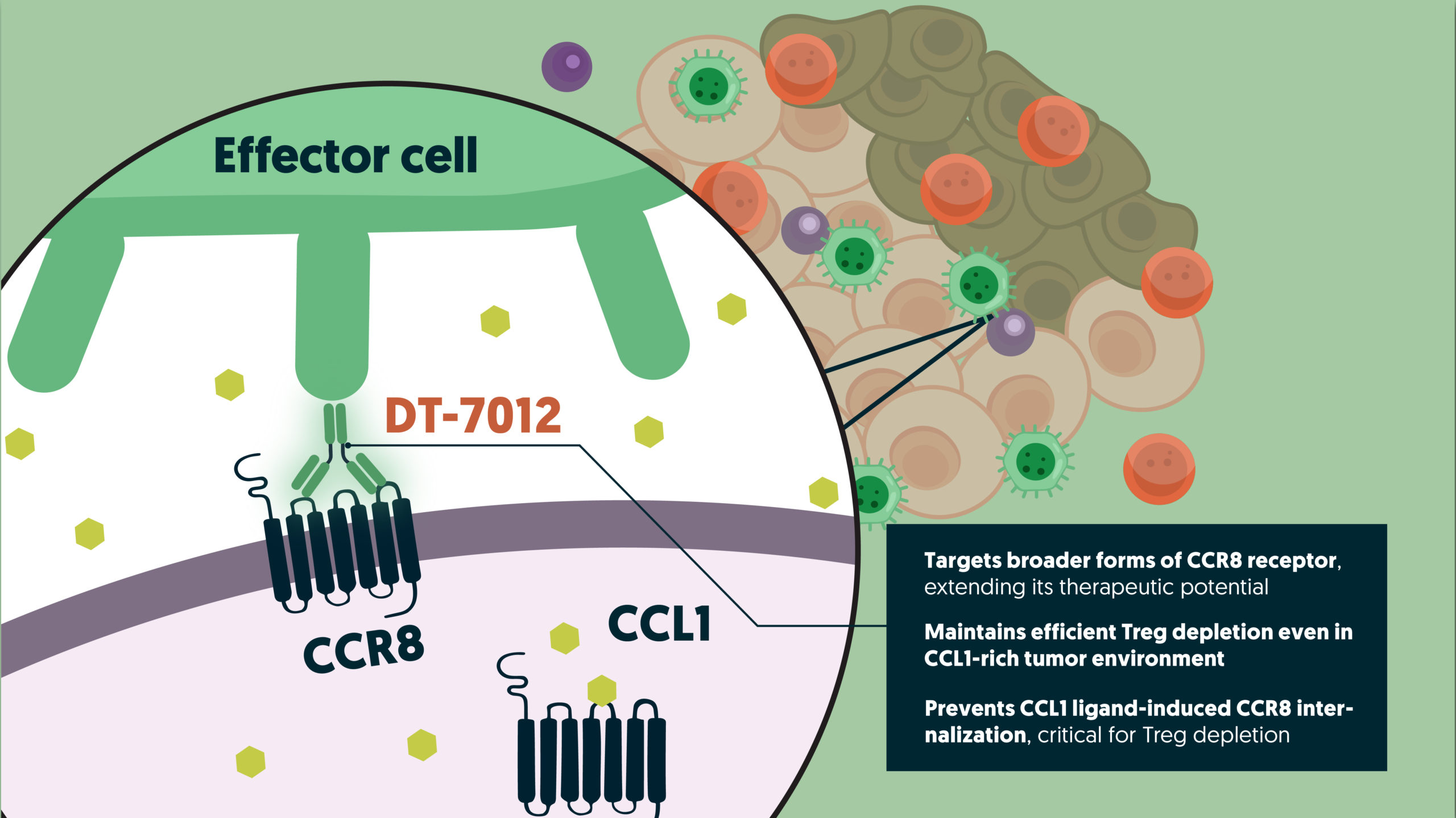
Moreover, this asset was designed to target broader forms of the CCR8 receptor compared to other anti-CCR8 clinical candidates. This extends its therapeutic potential to a wider range of immunosuppressive Treg subpopulations, thereby benefiting a larger population of patients.
Another standout feature of DT-7012 is its ability to prevent CCL1 ligand-induced CCR8 internalization, a critical factor for Treg depletion. By ensuring CCR8 remains at the membrane, DT-7012 potentiates depletion mechanisms, even in challenging CCL1-rich tumor microenvironments. This sustained Treg depletion is essential for effective cancer immunotherapy, conferring breakthrough therapeutic properties to this program.
CCR8: a superior target for Treg depletion, unlocking the immune system’s cancer-fighting abilities
C-C chemokine receptor type 8 (CCR8), is a GPCR family receptor, predominantly expressed by Tregs, which are crucial immunosuppressive cells. Activated by the CCL1 ligand, CCR8 draws Tregs to the tumor site, promoting an immunosuppressive environment that fosters tumor growth.
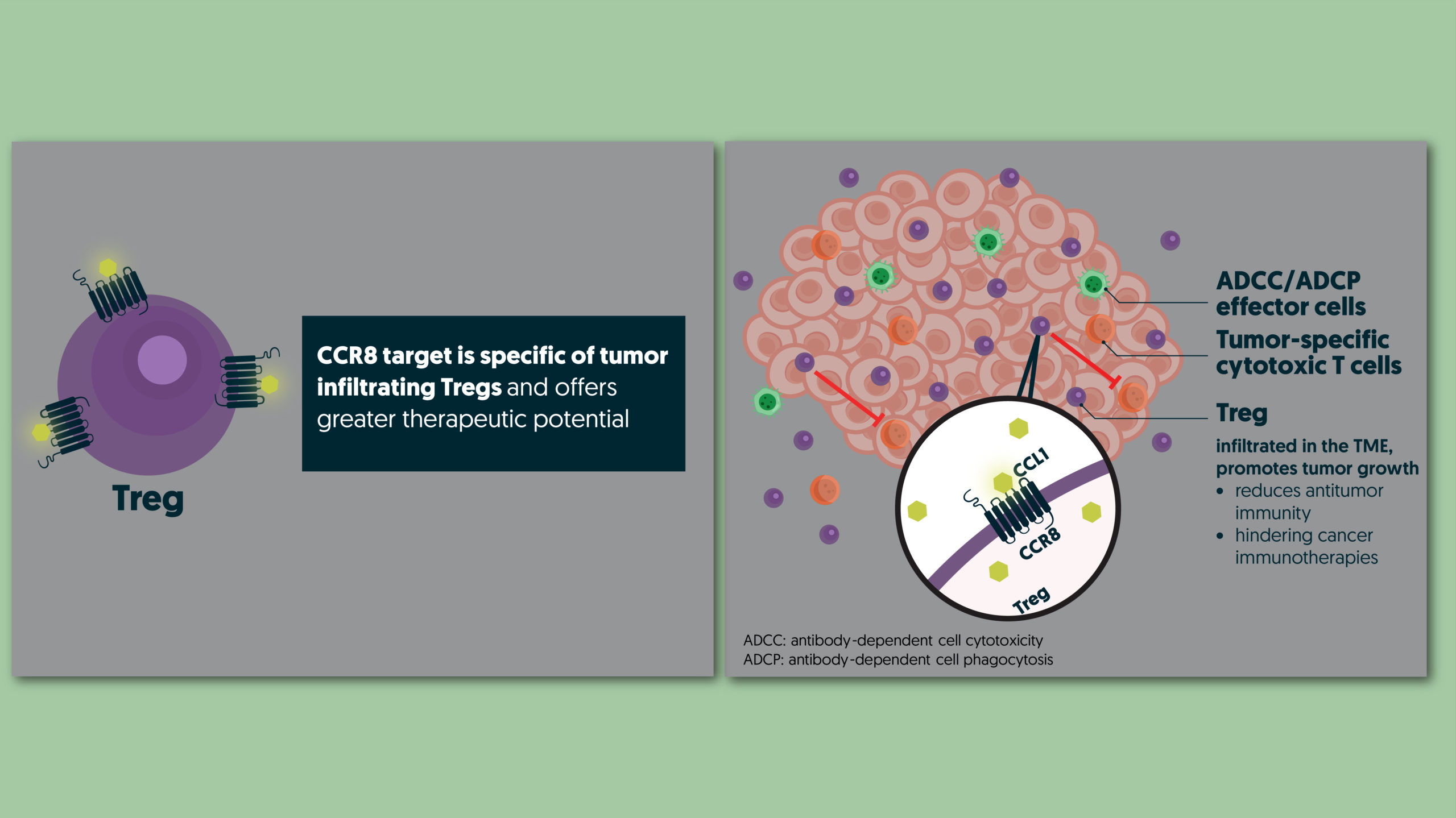
While immune-checkpoint inhibitors (ICI) have revolutionized cancer treatment, the tumor-infiltrating Tregs suppress immune response, leading to resistance against ICIs and compromising the efficacy of these treatments. This highlights the urgent need for new strategies to improve clinical outcomes for patients who do not respond to current treatments.
Strategic depletion of Tregs via CCR8, specifically expressed on tumor-infiltrating Tregs, is a highly promising approach to counter immunoresistance in the TME. DT-7012 is at the forefront of this innovative therapeutic strategy aiming to unlock the immune system’s cancer-fighting abilities and offer a powerful new therapeutic solution that can transform the lives of cancer patients worldwide.
Additional resources
• DT-7012 exhibits high-affinity to CCR8, potent effector functions, and selective depletion of CCR8+ Tregs in patient samples as well as a good safety profile.
• DT-7012 has differentiated and advanced properties compared to most of its competitors.
• Phase 1/2 has been approved and will be launched in Q2-2025.
Due to its high and relatively specific expression on tumor-infiltrating Tregs, CCR8 represents an attractive novel target to derive novel immunotherapies.
• At Domain, we successfully discovered and patent-protected a mAb library of several dozen of antibodies with distinct and differentiated binding and activity profiles.
• This collection constitutes a unique source for the development of a best-in-class well-differentiated anti- hCCR8 depleting antibody for the treatment of cancers.
• Based on promising profiles, a set of anti-CCR8 mAb leads with distinct characteristics have entered late optimization phase (humanization, enhanced cytotoxic activity (i.e., ADCC, CDC, ADCP), …). Cell line generation will be initiated in Q1-2023.